September 9, 2021 was a big day for the FDA – a year after a big day for the vaping industry as businesses both big and small scrambled to finish submitting the Premarket Tobacco Applications (PMTA...
Continue Reading

September 9, 2021 was a big day for the FDA – a year after a big day for the vaping industry as businesses both big and small scrambled to finish submitting the Premarket Tobacco Applications (PMTA...
Continue Reading
In case you missed it, the FY 2021 Omnibus Appropriations Bill, also simply referred to as the appropriations or spending bill, passed by Congress in December contained an unwanted gift for the vaping industry: an amendment to the PACT Act of 2009 to include a […]
Continue Reading
It’s been a month since the vaping industry was in a scramble to finalize and submit the required premarket tobacco applications (PMTAs) by the FDA’s September 9th deadline – and we’ve got to tell you, it has taken our small PMTA team here at White […]
Continue Reading
If you’ve been to the Puff Bar website recently, then you’ve probably already seen this message: “Puff Bar has ceased all online sales & distribution in the U.S. until further notice.” The site doesn’t offer any explanation as to why, although you can […]
Continue Reading
Ah, smoking and drinking — a duo as timeless as “singing and dancing,” “peanut butter and jelly,” or “Bieber and poor judgment.” Before most of us made the switch to e-cigarettes, the act of smoking went hand-in-hand with … well … whatever cocktail you chose. […]
Continue Reading
Since their introduction, electronic cigarettes have been used by many smokers as an alternative to smoking traditional tobacco cigarettes. Despite the many opposing views on the potential health benefits of switching from smoking to vaping, research has suggested that more and more people have successfully […]
Continue Reading
Nicotine gets a bad rap. Because it’s believed to be the addictive ingredient in tobacco cigarettes, and everyone knows that smoking kills millions every year, many people assume that nicotine is a dangerous substance. In extremely high doses, nicotine is indeed poisonous; however, in moderation, […]
Continue Reading
There are just a few more days until the unofficial start of summer! Here at the White Cloud HQ, we’re looking forward to spending our weekends at our beautiful beaches and parks, enjoying time spent outdoors under the Florida sun. While we’ve discussed what sorts […]
Continue Reading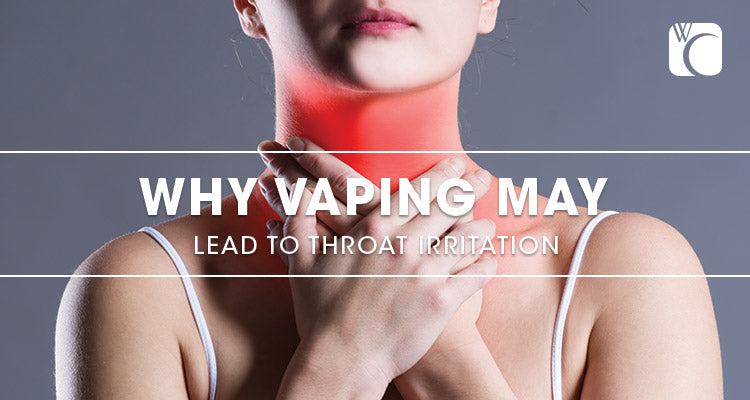
That little tingle in the back of your throat when you light up a tobacco cigarette is a feeling that many new vapers are expecting when taking their first puff of an e-cigarette. But a chronic sore throat can quickly suck the fun out of […]
Continue ReadingYour bag is currently empty.